



















1) What is the conjugate base of HCO3−? Express your answer as a chemical formula. 2) What is the conjugate acid of HPO32− ? Express your answer as a chemical formula. 3) Among three bases, X−, Y−, and Z−, the strongest one is Y−, and the weakest one is Z−. Rank their conjugate acids, HX, HY, and HZ, in order of decreasing strength. Finding conjugate acid and conj. base of HCO3-. In parts (c) and (d) of problem 11.1 from the textbook, we are asked to write the formula of the conjugate base of HCO3- (book answer: H2CO3) and the conjugate acid of HCO3- (book answer: CO3^2-). thus the conjugate base of any compound is the compound after the removal of H+ from them respectively The conjugate base of acid HCO3- is (CO3)^2- The conjugate base of H2O is OH- The conjugate base of HCO 3-is CO 3-2, which is the carbonate ion.. To determine the conjugate base of a substance, you remove one hydrogen ion. It's... See full answer below. So, in order to determine the conjugate acid for a given base, all you have to know is that "base" + H^(+) -> "conjugate acid of that base" In your case, the base is hydrogen carbonate, or HCO_3^(-). If you write the equation you'll get HCO_3^(-) + H^(+) -> H_2CO_3 Carbonic acid, or H_2CO_3, will be the conjugate acid of hydrogen carbonate. Most of the conjugate acids you'll ever need to know : HCO3- : HCO3− Hydrogencarbonate (bicarbonate) ion. CO32− Carbonate ion ===== HFSbF5 Fluoroantimonic acid SbF6− Hexafluoroantimonate ion. HCl... Create your account. What is the conflict of the story of sinigang? Conjugates always differ by one H+. All other trademarks and copyrights are the property of their respective owners. H2CO3 is the acid (releases H+) ---> (HCO3)-1 is its conjugate base (will take that H+ back) H2O is the base (takes H+ ion) The conjugate base of HCO3- is CO32-. Conjugates always differ by one H+. A conjugate base has one fewer H+, while a conjugate acid has one more H+. What is the conjugate base of HCO3-? Explanation. What is the conjugate base of HCO3-? Answer. The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-. HCO3- is a conjugate acid, H 2 CO 3. Answer to Part B What is the conjugate base of HCO3 ? Express your answer as a chemical formula. View Available Hint(s) - AED O ?
[index] [7718] [3167] [9256] [2846] [5562] [441] [3953] [5062] [1417] [4724]
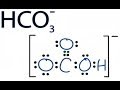
A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl... In this video we will learn the difference between strong and weak acids and bases. Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks If you want to understand acid-base analysis, then you need to understand buffers. In this short video, you will learn everything you need to know. Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... Pyruvic acid is placed in water at physiological pH (7.3). Under these conditions, which species will dominate; the conjugate base or the conjugate acid? The... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com A step-by-step explanation of how to draw the HNO3 Lewis Structure (Nitric Acid). The HNO3 Lewis structure is best thought of as the NO3 with an H attache... This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. It explains the concept, compo...
Copyright © 2024 hot.sportsfootball.site